![]()

by Phil Harper
On May 24th, the anonymous Twitter account JikkyLeaks claimed that data contained inside the massive Pfizer Documents release shows their vaccine had close to zero efficacy even when it launched. There is some correcting of the raw data required to reach this conclusion, but the raw data alone hints at an efficacy of 53%. The data contradicts Pfizer’s published claims the vaccine was 95% effective, a claim still published on gov.uk domains. The original data and specifically the “95% efficacy” claim was key to getting the vaccines onto global markets around the world. If Pfizer reached this conclusion dishonorably, there could be sizable implications.
At least one other researcher has publicly reached the same results using data contained in the court-ordered Pfizer documents. I have already verified that the data exists, and using very simple public code, that the numbers add up to what Josh and Jikky claim. Understanding what they mean, and the potential implications is what I will now address here.
I reached the same results independently. @Pfizer, you got some splainin' to do… https://t.co/vpg49dGExA
— Josh Guetzkow (@joshg99) May 24, 2022
Not only has the specific claim been entirely ignored, which we might reasonably expect, but the existence of the documents themselves has also been entirely ignored. Against a backdrop of silence, this article is my attempt to unpack this data in an accessible way. To do that requires understanding how so many regulators around the world became dependent on ‘the word’ of Pfizer, a company with a proven track record of causing “false claims to be submitted to government health care programs”
‘The Rolling Review’
In November 2020, Pfizer started to make its successes with the vaccine known to the media. Towards the end of the month, they published a press release stating their vaccine was 95% effective. In the release, they said they planned “to submit within days to the FDA [and other regulators] for Emergency Use Authorisation.” With their guard down, the media didn’t hold these claims to account. Instead, and perhaps understandably, they choose to jump for joy. The regulators were pleased too; just two weeks after Pfizer’s press release, the UK regulator announced it had approved the Pfizer vaccine and that rollout would start within a week. Reporting the announcement, the British Medical Journal made note of something quite remarkable:“No results from the trial have yet been published in a peer-reviewed journal.”
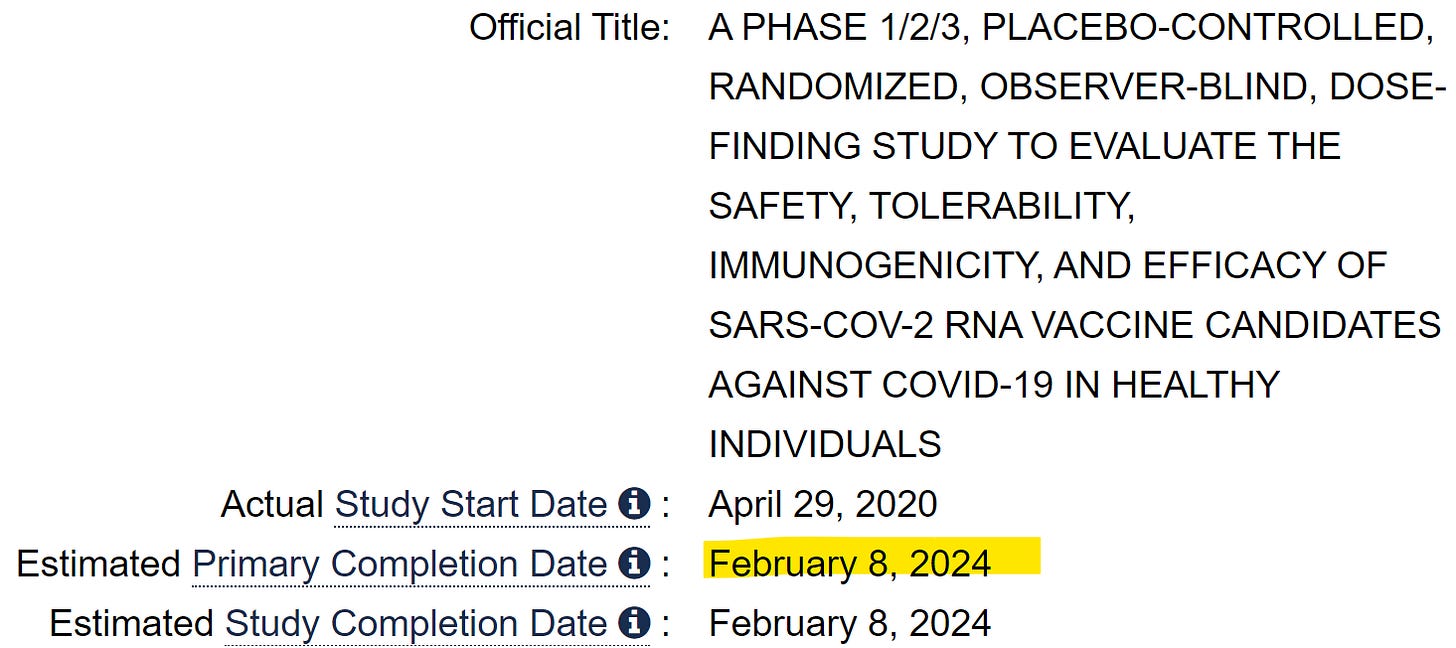
It was true. The Phase 3 trials of the vaccine were still ongoing, but the vaccine was now approved for use in the UK. The trials are still ongoing according to the registered protocol, which says they’re not scheduled to be complete until February 8, 2024. So how did the vaccine come to be approved before the full completion of Phase 3 trials? According to an FOI request, and a public article, the UK regulator addressed the question and said it had used a ‘rolling review’ process to approve the Pfizer vaccine. The review was done “as the packages of data become available from ongoing studies”.
Pfizer’s BNT162b2SA study protocol shows study completion Feb 8th 2024
Whatever that regulatory process was, it wasn’t exactly public. To make matters worse, the trial had problems with impartiality and data sharing, which, two years later, is something that still frustrates Peter Doshi at the British Medical Journal.“Pfizer’s pivotal covid vaccine trial was funded by the company and designed, run, analysed, and authored by Pfizer employees. The company and the contract research organisations that carried out the trial hold all the data. And Pfizer has indicated that it will not begin entertaining requests for trial data until May 2025”
Why didn’t we have the data public made public immediately? Describing the regulatory process, Pfizer CEO Albert Bourla said “Thirty of our people couldn’t sleep for five days… then the FDA would start. Then thirty FDA people would not sleep for five days.” He was triumphantly describing the process with Klaus Schwabb at the World Economic Forum in May of this year [22:28s]. It normally takes 10 years to develop and regulate a vaccine, but in 2019, the whole process was done in 10 months. It’s worth noting, that had Pfizer wanted to slip an ineffective product past regulators, this might be a good way to do it. An exhausting back and forth of complicated and important trial data, all done in an impossibly short period, when everyone is desperate for a solution. What could possibly go wrong?
Whatever breakneck process was taking place at the FDA, things were even quicker at the UK regulator, because they approved the vaccine even sooner. They got it out of the door ten days earlier, in what looked like a political competition over who could approve the vaccine soonest. At the tail end of his administration, ruffled Trump officials demanded meetings with the regulator ‘to discuss timelines’ over the vaccine’s approval.
By skipping over the public phase which normally happens by publishing the studies in medical journals, oversight from the broader medical community had effectively been torched. It was now critically important that the information Pfizer gave to the regulators was accurate and gave a fair representation of the vaccine. So, was the data accurate? Did the incredible regulatory pace mean things were missed? And what exactly happened in that strange regulatory process?
A document called the “FDA Briefing Document“, prepared by Pfizer and published by the FDA on December 10th 2020 can give us some clues. The document is somewhere between a sales pitch and an executive summary of the trial data. The same information was presented by Pfizer to multiple regulators all over the world. It contained a critical piece of data that underpinned the “95% efficacy” line that Pfizer had been touting in the weeks before.
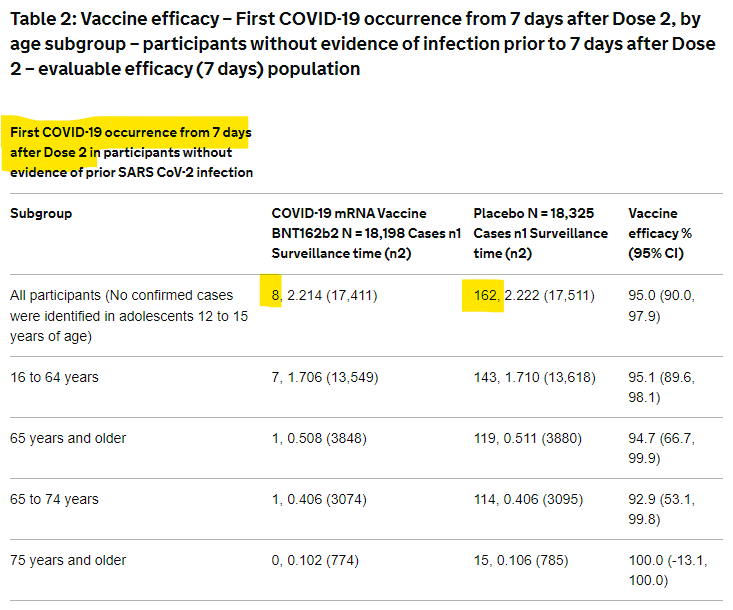
When June Raine and her team of UK regulators “robustly and thoroughly reviewed [Pfizer’s data] with great scientific rigour”, the part showing it to be 95% effective was of crucial importance. She said so herself, saying they had “looked at how the vaccine protects people from COVID-19”. So, Table 2, presented below, was mission-critical because it was the basis for the 95% efficacy claim. But there’s more to this claim than meets the eye. It marks the beginning of Pfizer misleading the public with their data.
Data from Pfizer Phase 3 was published on the UK Gov website somewhere around June 9th. The information had been shared with multiple regulators in December 2020.
A Very Common Trick
Essentially, what the table shows is that of the trial participants taking the vaccine, only 8 of them tested positive for Covid-19 within 7 days. Of the trial participants taking the placebo, 162 people tested positive for Covid-19 within 7 days. The difference between those two outcomes is 154, which they use to calculate a 95% efficacy. How do they get there? The vaccine ‘reduced’ Covid-19 outcomes by 154, out of a total of 162, and since 154 is 95% 162, they say that’s “95% efficacy”. Does that feel correct? We’ll return to it shortly.
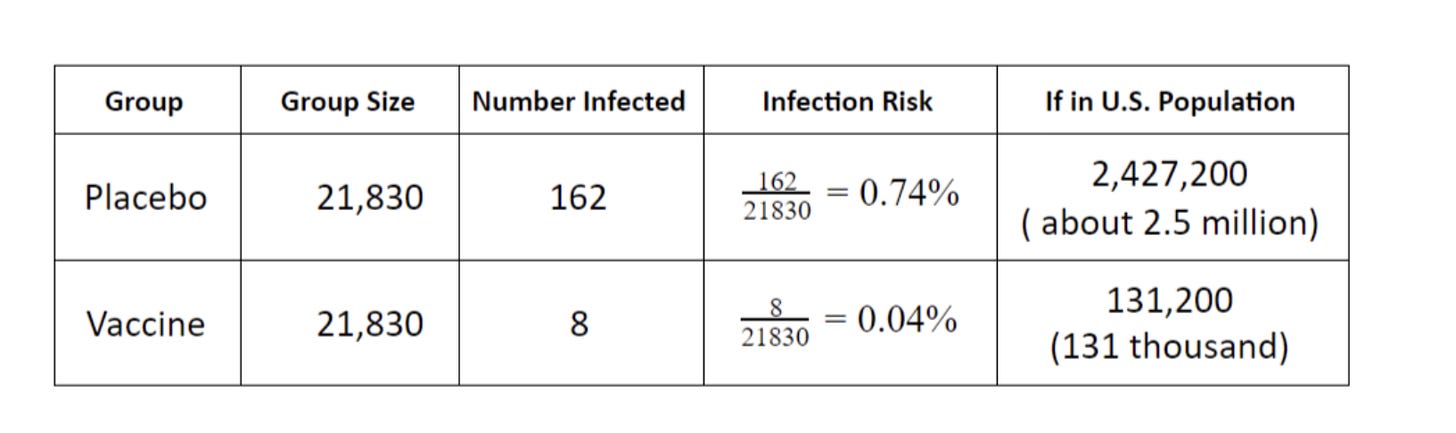
“Assume the U.S. population had the same infection risk as the vaccine group. How many people would you expect to become infected?” asked the NY Times. This table showed the answer. Link
Nicely demonstrating how this data had been understood is an article from the New York Times which was published not long after Pfizer’s data had been released. As part of a revision guide for students, the article posted a table calculating how many people would be infected if we vaccinated everyone in America. Using the exact figures from Table 2, they calculated there would be just 131,200 infections across the entire United States. In reality, between June 2021 to June 2022, there were 52 million cases during a period that built towards 67% of the population being double vaccinated. So the discrepancy between the marketing and reality is massive, so what happened? Was the data wrong, or were they misleading people over what the data really means?
[the_ad id=”157875″]
The first part of their trick is rooted in the gulf between what they hope is understood, and what the data really means. So we’re now going to unpack what the data means and see if that reality matches up to what we all think it means. This ‘doctoring of the data’ trickery is so rampant that its corrosive influence is rarely even acknowledged. It’s done so widely, at every stage of a drug’s development, that our understanding of these medical products is hopelessly and deliberately poisoned before these companies even start manipulating the underlying data. Before we can understand the ‘unacceptable’ form of manipulation that JikkyLeaks claims to have uncovered, we’ve first got to understand the manipulation that’s accepted as normal. Spoiler: what’s accepted as normal, is not normal.
Absolute or relative “efficacy”
Crucial to understanding the sleight of hand here is understanding what is meant by “efficacy”. I think it’s best imagined as the famous glass prism picture but in reverse. A world of diverse, complex, contradictory colours of data fire through a prism of glass, emerging out of the other side as “95% efficacy”.
The phrase has a ‘two-tier’ meaning; a public one designed to be simple and reductive, and a private one which implies clear qualifications by ‘those in the know’. This dual meaning makes the word incredibly useful because it placates two important but distinct groups of people; it can fool the public into believing the medicine is ‘effective’, whilst its implied qualifications keep ‘those in the know’ from being upset about its liberal use. Like a chameleon, it changes its colour depending on its context.
So how is it understood by the public? Well, Pfizer‘s “BNT162b2” product is understood by the public as a vaccine. It is described that way explicitly by the media, and it’s marketed that way by Pfizer themselves. Therefore, the public understands its ‘efficacy’ to mean its ability to create immunity to disease, in this case, Covid-19. So when Pfizer ran with their “95% effective” line, they know that what the public ‘hear’ is that the vaccine stops you from getting Covid-19 with 95% certainty. This is what Pfizer want the public, and even doctors, to understand.
But the medical world has much more precise discussions about what exactly the “95% efficacy” relates to. There are many factors that feature in “efficacy”, and they’re all concealed in that one singular word. Does it create immunity? Is it lasting immunity? How long does this ‘immunity’ last? Is it a relative risk reduction? An absolute risk reduction? What’s the confidence interval? Does it only reduce symptoms? By how much does it reduce the symptoms? How are we measuring a reduction in symptoms? Anywhere that social media permits, these questions are being debated right until this very moment.
The most critical component of “95% efficacy” is how it’s calculated. To get there, we’ve ignored the vast majority of the outcomes from the trial. Have a look again at Table 2. The overwhelming majority of people on the trial did not become sick with Covid-19, even if they didn’t receive the vaccination. If you look, you’ll see that out of 18,325 people in the placebo group, only 162 went on to get sick with Covid-19. That’s only about 0.88%. We can use this data to say the human immune system had a “99.2% efficacy” in stopping Covid-19? But that’s higher than the efficacy of the vaccine?! So what is going on?



Notice the crickets from our mainstream media?
That includes all of them, btw.
Fox News included.
There’s been a move to kick Newsmax off the cable lineups, as Newsmax does, on occasion, speak about this.
Altho Pfizer cannot get an FDA approved vax into Americans, they still push for babies to be vaxxed.
And, altho these vaccinations are only good for a few days while babies are 99.999% likely to survive covid, they push babies to be vaxxed.
Without spending a dime on sales adverts, Pfizer has become super-rich off the pure profits from this vax.
No one can trust the vaccines. The numbers of adverse events and deaths make the vaccines, all of them, questionable and not safe.
https://twitter.com/FiveTimesAugust/status/1540065368026275846?t=Rhsq1KJd_ZrOk5f-F1qYLQ&s=19